Adverse Event Tracking
Used by the world’s largest medical device and pharmaceutical companies, ExtraView is the foundation for global capture, analysis, and resolution of adverse events related to medical device, treatment and diagnostic systems.
ExtraView Adverse Event is in use today at some of the world's largest medical device and pharmaceutical companies to track quality issues under strict compliance with the US FDA 21 CFR Part 11 standard for electronic records and electronic signatures.
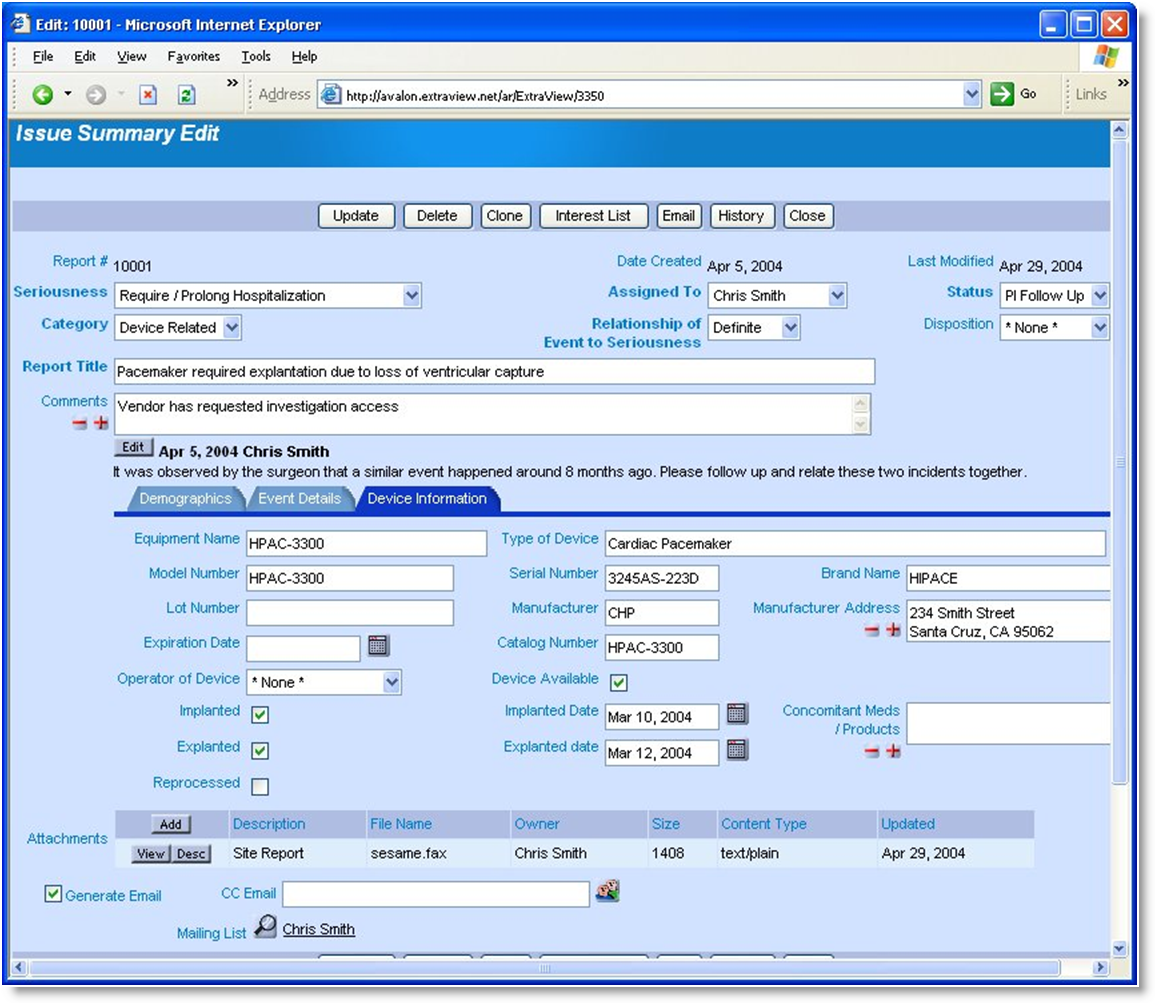
Beyond exceptional scalability and flexibility, ExtraView is unique in the ability to authenticate users at key points in the workflow while auditing all activity related to fields and issues in the database. ExtraView also provides customizable PDF templates designed to generate standard forms for FDA submission.
- Login to post comments
